Regulatory Affairs
When people get sick they rely on modern medicine to make them better and this is done without a second thought because they know that the manufacturers have spent years developing their products to ensure their effectiveness and safety. The animal health industry also invests huge resources in developing, testing and manufacturing safe, effective veterinary medicines and Europe has one of the world’s most stringent licensing systems for controlling medicines (see Bringing veterinary medicines to market).
EFFICIENT REGULATION
IFAH-Europe's vision is for a regulatory process that can deliver a true single market for these products via a single European marketing authorisation for all animal health products.
Adherence to common standards at a global level is also important and as such IFAH-Europe promotes a Global Regulatory Strategy for Veterinary Medicines and is active in the VICH, a trilateral (EU-Japan-USA) programme aimed at harmonising technical requirements for veterinary product registration.
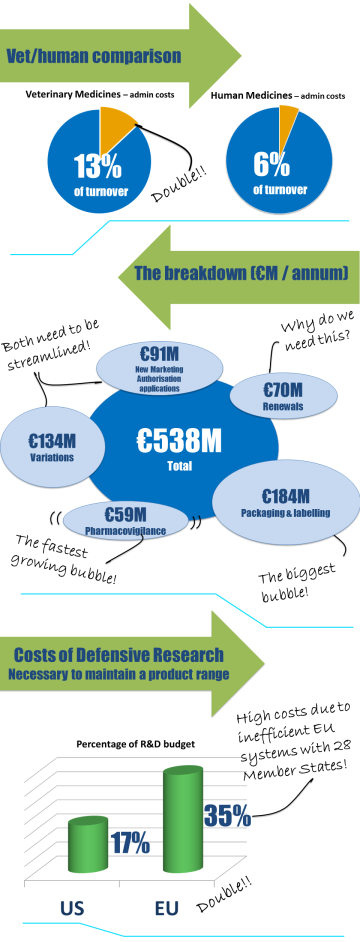
The links below give more detail on how a single European marketing authorisation could work and the benefits of a more harmonised system for Europe.
A single market for veterinary medicines
The road to a single EU market for veterinary medicines began with the first European Directive in 1981. During the last 30 years significant milestones have been achieved in realising this objective through significant updates and revisions of the legislation. To assist with the preparation of the European commission’s impact assessment on proposed revisions to EU legislation on veterinary medicinal products, IFAH-Europe prepared a comprehensive Impact Assessment Data Package.
IFAH-Europe now has one vision: to complete the journey to a single market in veterinary medicinal products. The ongoing review of the legislative framework is an opportunity to deliver that goal. The European Commission published proposals for revised legislation governing veterinary medical products in September 2014. The draft legislation is currently being discussed by the European Parliament and the Council.
Our position on the proposed Regulation for veterinary medicines
Common goals
- A single market for veterinary medicines in Europe.
- The availability of safe, efficacious veterinary medicines of appropriate quality to protect both animal health and public health.
Objectives
- Enhanced availability of veterinary medicines
- Efficient use of resources
- Simplified yet robust regulatory system